Our laboratory benchmarking
Since the 1990s, benchmarking has been an established management tool to determine one’s competitive position, identify areas for improvement and support the development of a laboratory strategy. In addition, benchmarking helps to design an effective laboratory optimization program or to give new impetus to an existing program through additional improvement ideas and external benchmarks or to measure progress to date.
Available modules

360° Benchmarking

Cost-effectiveness benchmarking

Lead time benchmarking

Reliability Benchmarking

Top-level benchmarking

Productivity benchmarking

Compliance Benchmarking

Benchmarking for resource efficiency
About benchmarking
The worldwide unique benchmarking especially for pharmaceutical quality control laboratories
In addition to the classic comparison of key figures, PharmaLab Benchmarking goes two levels deeper: in a best practice comparison, laboratory processes and practices are compared and the most promising areas for improvement are identified. Finally, as part of the learning needs analysis, the prerequisites for implementing the opportunities for improvement are determined.
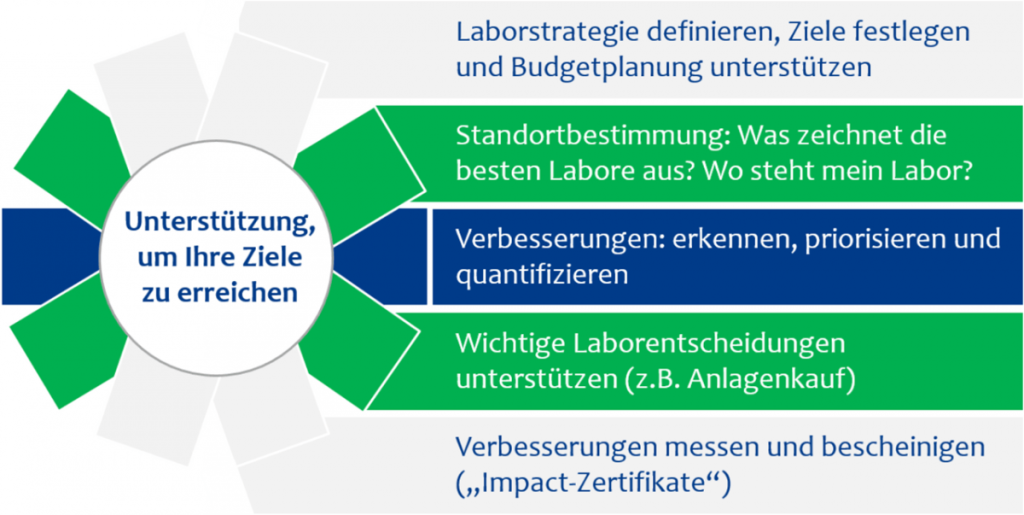
BENEFITS FOR LABORATORIES
Unique in the world
The world’s only benchmarking specifically for pharmaceutical quality control laboratories
Unique in the world
Robust comparison of key figures through proprietary normalization method (“apples with apples”)
Best practice comparison
By comparing over 100 laboratory best practices, the analysis goes beyond pure key figures
Systematic analysis
Systematic analysis of weak points and “blind spots” in your laboratory
Fact-based comparison
Fact-based comparison with the best quality control laboratories
Concrete starting points
Concrete starting points for improving laboratory processes and practices
Skills analysis
Analysis of the skills your employees need to implement improvements
Modular design
Fast and focused implementation thanks to modular benchmarking structure
Little effort
Simple process and significantly less effort than in-house analysis
Comprehensive results report
PharmaLab Benchmarking Report
- Comparison of over 200 key performance and process indicators along the "magic triangle": costs, speed and quality/compliance.
- "Maturity Assessment" with over 200 laboratory best practices, which are responsible for the characteristics of the key figures.
- Learning needs assessment with over 50 skills needed to successfully improve lab processes and practices.
200+
Key figures
500+
Best Practices
60+
Skills

PROCESS

1. preparation
During the one-week preparation phase, the key requirements for the benchmarking are defined. This includes determining the project team and planning the deadlines.
2. data collection
Data collection takes around 6-8 weeks. In particular, all essential data for benchmarking is collected and surveys are carried out using a questionnaire.
3. report generation
Finally, the benchmarking report is prepared and the results and implications regarding the improvement topics are discussed and prioritized in a workshop.
REFERENCES

Kristine Oelkers
“I was impressed by the benchmarking methodology: the insights you get are simply fantastic.”

Prof. Dr. Harald Hungenberg
“Geniu’s benchmarking is unique worldwide and offers significant benefits for laboratories.”

Dr. Armand Farsi
“Benchmarking is much more than an ordinary comparison of key figures: it reveals specific potential for improvement.”
FREQUENTLY ASKED QUESTIONS

Can only parts of the benchmarking be examined?
![]() The modular structure of the benchmarking allows not only a quality control laboratory unit to be analyzed as a whole, but also a laboratory unit or a performance dimension individually.
The modular structure of the benchmarking allows not only a quality control laboratory unit to be analyzed as a whole, but also a laboratory unit or a performance dimension individually.
When can benchmarking be started?
![]() Benchmarking can be started at any time. Whether in January to set new goals for the year or in October to support budget preparation: We can usually conduct the kick-off meeting within a few days.
Benchmarking can be started at any time. Whether in January to set new goals for the year or in October to support budget preparation: We can usually conduct the kick-off meeting within a few days.
How is the data collected?
The process and performance indicators are collected using a data collection form, while the best practice comparison and learning needs analysis are carried out using questionnaires. The latter are typically completed by the head of quality control and their managers.
How much effort is involved in benchmarking?
The data collection effort depends on the individually selected scope of the benchmarking and the availability of data:
- For one sub-area/dimension: 1-3 days of work spread over 1-3 weeks
- For the entire quality control area: 3-5 days of work spread over 3-6 weeks
How much experience does Geniu have in carrying out benchmarking?
![]() In over 100 pharma benchmarking studies, we have built up extensive experience in how to develop robust benchmarking. Components such as the proprietary normalization method and the precise definitions of data collection are the result of these many years of experience.
In over 100 pharma benchmarking studies, we have built up extensive experience in how to develop robust benchmarking. Components such as the proprietary normalization method and the precise definitions of data collection are the result of these many years of experience.
How is the comparability of the laboratories ensured?
![]() Quality control laboratories differ. Therefore, benchmarking compares laboratories in groups of structurally similar laboratories. In addition, differences in the product and test portfolio within each group are taken into account using a proprietary normalization method.
Quality control laboratories differ. Therefore, benchmarking compares laboratories in groups of structurally similar laboratories. In addition, differences in the product and test portfolio within each group are taken into account using a proprietary normalization method.
How is the confidentiality of the data ensured?
![]() Data confidentiality is essential for benchmarking. Therefore, Geniu’s benchmarkings follow the strict “Good Benchmarking Practices” that fully protect your data. In addition, a confidentiality agreement is concluded.
Data confidentiality is essential for benchmarking. Therefore, Geniu’s benchmarkings follow the strict “Good Benchmarking Practices” that fully protect your data. In addition, a confidentiality agreement is concluded.